
Sinolyte® TDT
Cas no 201419-80-9
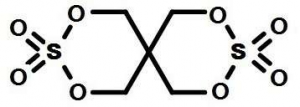
Chemcial Name: Pentaerythritol Bicyclic Sulfate ; 2,4,8,10-Tetraoxa-3,9-dithiaspiro[5.5]undecane 3,3,9,9-tetraoxide
Molecular structure:
Description
The sulfate group of the bicyclic sulfate compound can be reduced by itself by accepting electrons from the anode surface during charging, or can react with previously reduced polar solvent molecules, thereby affecting the characteristics of the SEI layer formed thereon. Anode surface. For example, compared with polar solvents, bicyclic sulfate-based compounds containing sulfate groups can more easily accept electrons from the anode. For example, before the polar solvent is reduced, the bicyclic sulfate-based compound can be reduced at a lower voltage than the polar solvent.
Bicyclic sulfate-based compounds include sulfate groups, and therefore can be more easily reduced and/or decomposed into free radicals and/or ions during charging. Therefore, free radicals and/or ions can be combined with lithium ions to form a suitable SEI layer on the anode, thereby preventing or minimizing the formation of products obtained by further decomposing the solvent. Bicyclic sulfate-based compounds may form covalent bonds with, for example, the carbonaceous anode itself or various functional groups on the surface of the carbonaceous anode, or may be adsorbed on the surface of the electrode. Compared with the SEI layer formed by only organic solvents, the modified SEI layer with improved stability formed by this combination and/or adsorption can be more durable even after long-term charging and discharging. During the lithium ion intercalation in the electrode, the durability-modified SEI layer can more effectively prevent the co-intercalation of organic solvents that solvate lithium ions. Therefore, the modified SEI layer can more effectively prevent the direct contact between the organic solvent and the anode to further improve the reversibility of the intercalation/deintercalation of lithium ions, resulting in an increase in the discharge capacity and the life characteristics of the manufactured battery. improve.
Moreover, due to the inclusion of a sulfate group, the bicyclic sulfate-based compound can coordinate on the surface of the cathode, thereby affecting the characteristics of the protective layer formed on the surface of the cathode. For example, the sulfate group may coordinate with the transition metal ion of the cathode active material to form a complex. The composite can form a modified protective layer with improved stability, and even after long-term charging and discharging, the protective layer is more durable than a protective layer formed only by organic solvents. In addition, the durability modified protective layer can more effectively prevent the co-intercalation of organic solvents that solvate lithium ions during the intercalation of lithium ions into the electrode. Therefore, the modified protective layer can more effectively prevent the direct contact between the organic solvent and the cathode to further improve the reversibility of the insertion/deintercalation of lithium ions, thereby improving the stability and life characteristics of the manufactured battery.
In addition, bicyclic sulfate compounds have a structure in which two rings are connected in the form of a spiro ring. Therefore, the bicyclic sulfate-based compound may have a larger molecular weight than the usual sulfate-based compound, and thus may be thermally stable.
For example, a compound based on a bicyclic sulfate may form an SEI layer at the protective layer at the anode surface or the cathode surface, and may promote enhanced life characteristics, stability, of lithium batteries manufactured at high temperatures due to improved heat.
Contact Us Now!
If you need COA, MSDS or TDS, please fill in your contact information in the form below, we will usually contact you within 24 hours. You could also email us at info@sinocurechem.com during working hours ( 8:30 am to 6:00 pm UTC+8 Mon.~Sat. ) or use the website live chat to get prompt reply.
