Because of the principle of entropy increase.
The second law of thermodynamics (the principle of entropy increase) tells us: for an isolated system, the system always develops in the direction of maximum chaos (a state of high entropy).
☆Completely pure substances are difficult to maintain
The concept of pure matter can only exist in a chemical sense, and completely pure matter cannot be found in the macroscopic world. The entropy of completely pure matter is necessarily extremely low. Without human intervention, it can also spontaneously develop toward a high-entropy state and become impure.
Suppose you get a bottle of completely pure substance through the hand of God (some way), you are like a treasure, carefully sealed, expecting media people and scientists from all over the world to come to smooth the threshold of your home to visit and Research.
But unfortunately, your container itself is also a kind of substance. The substance in the container and the inner wall of the container are in direct contact with each other, which will cause substance exchange. The molecules or atoms of the container material will enter the pure substance. If there are impurities, it will be impure.
This “damn” atoms and molecules are always in irregular thermal motion. They have to have sororities with other substances to break your illusion that they can remain pure forever.
In addition, your “pure matter” itself will “self-revolution”, undergo chemical changes, and be transformed into things in other camps. These substances may undergo decomposition reactions on their own, or even self-assemble into other things, and become impurities.
Just as you should never expect that a dirty table will automatically become clean, neither should you expect impure substances to become pure spontaneously. The price of becoming orderly is energy.
☆Purification is a chore: the higher the purity requirement, the more difficult it is
Impurity removal is to remove the parts that you don’t need in the matter. This process will reduce the entropy of the matter, which is to make it more “ordered”.
This requires a “price” and needs to provide energy to this system. All impurity removal or purification processes require energy! What’s more interesting is that if the purity you need is higher, the technical difficulty of purification will be more difficult, and the energy consumption will increase exponentially.
Chemists have long summarized a set of magic weapons for purification, filtration, extraction, evaporation and concentration, distillation, electrolysis, crystallization, reverse osmosis, ion exchange, chromatography… These magic weapons are like chemists’ “crabs”. With these tools, chemists can clean up the contents of their bottles.
Either these physical methods or chemical methods, without exception, consume energy and resources!
However, these purification methods often have limitations. For example, for impurities in the liquid, distillation can be used to remove them, but this method is often limited. For example, only the use of distillation to purify alcohol (ethanol and water) Mixture). When the ethanol concentration reaches 95.63%, ethanol and water form an azeotrope, which cannot be further purified. If the purity of 99% is to be achieved, other methods must be adopted.
At the same time, the method of rectification is only effective for liquid-liquid separation (separation of liquid impurities in the liquid). If you want to use rectification to separate the diamonds in the sand, it is a idiot. To eat crabs, different tools are used for different positions, and sometimes there are various tools to match. Similarly, different purification methods are required to separate mixtures in different states, and sometimes multiple purification methods are used to achieve the purity you want.
In this way, removing impurities is really a very technically difficult task, but it is also normal. If you want to eat crabs, these necessary efforts need to be paid.
It is relatively easy for chemists to tinker with the substances in bottles and cans, but it is really a headache for chemical engineers to face the products in the steel behemoth in front of them. It can be said that the core of the problem for chemical engineers is not how to make products (the chemist has already opened up in the laboratory in this step), but how to separate and purify these products from reactants, by-products, and solvents. .
The chemical operation of impurity removal has elevated it to the status of “separation engineering” in the chemical industry.
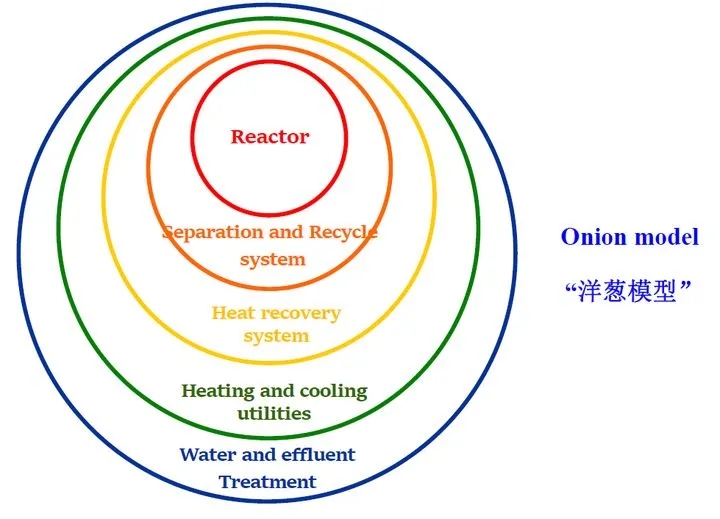
This is the famous “onion model” in chemical design. Separation engineering is the second core content of chemical design. It is one of the most important unit operations in the production process of petrochemical, organic chemical, biochemical, fine chemical, pharmaceutical and other industries. one.
Generally, the separation device accounts for 50% to 90% of the investment in the infrastructure of the chemical plant, and the energy consumption accounts for 30% to 50% of the entire process. It can be said that a large part of the investment and daily consumption of the chemical plant are The development and application of separation has also received increasing attention on a global scale.
Chemical plants often have a purity target, and the purification will not continue until a certain purity is reached, because pursuing purity will cost more. Purification from 95% to 99% is much more difficult than purification from 50% to 90%, because when the impurity content is lower, the separation method used tends to be more refined and the consumption will be greater, so the more The more difficult it is later.
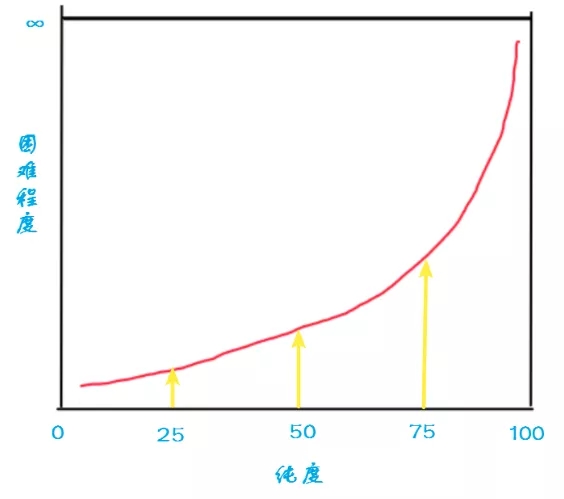
I drew a schematic diagram, the higher the purity of the target, the more difficult it is to purify
An analogy: Purification is like finding traitors from the collective;
Refined to 99%, there is 1 traitor among 100 members;
Refined to 99.9%, it is equivalent to 1 traitor in 1,000 members;
Refined to 99.99%, which is equivalent to 1 traitor among 10,000 members;
Refined to 99.999%, it is equivalent to 1 traitor with 100,000 members;
… …
The higher the purity requirements you need, the larger the scope of this collective, and further purification, you must find the traitor in a larger scope.
What is even more annoying is that during the purification process, other traitors may come in in troubled waters; (impurities introduced in the purification process)
It turns out that members of the collective may also betray (changes of pure material itself).
If it is purified to 100%, then the degree of difficulty must reach infinity, and it may only be done by the hand of God.
☆Classification of chemical purity to cure your “perfectionism”
Don’t pursue purity excessively!
In chemical experiment science, there are strict classifications of impurity content. The reagent specifications are basically divided according to purity (the amount of impurity content). There are high purity, spectral purity, benchmark, spectroscopic purity, superior grade purity, analytical purity and chemistry. There are 7 kinds of pure grades, and the most well-known are the “Four Great Pures”. Namely superior grade, analytical grade, chemical grade and experimental reagents.
(1) GR: Guaranteed reagent, also known as first grade or guaranteed reagent, 99.8%, this reagent has the highest purity and the lowest impurity content. It is suitable for important and precise analysis work and scientific research work, using green bottles sign.
(2) Analytical grade (AR), also known as secondary reagent, has a high purity of 99.7%, which is slightly inferior to the superior grade. It is suitable for important analysis and general research work and uses a red bottle tag.
(3) Chemically pure (CP), also known as third-grade reagent, ≥99.5%, with a large difference between purity and analytical purity. It is suitable for general analysis in industrial, mining and schools. Use blue (dark blue) labels.
(4) Laboratory reagents (LR: Laboratory reagent), also known as four-level reagents.
In order to meet some of the more special requirements, manufacturers will also make more pure reagents. For example, the reagents used in chromatograms need to be spectrally pure. Reagents whose purity is much higher than the superior grade are called high-purity reagents (≥ 99.99%). There are also ultra-pure reagents (impurity content is less than 1/1000000 ~ 1/1000000000), in addition to some relatively small ultra-pure classification.
For example: ICP-Mass Pure Grade: The content of most impurity elements is less than 0.1ppb, which is suitable for the daily analysis work of plasma mass spectrometer (ICP Mass). Atomic Absorption Spectroscopy Pure Grade Reagent (AA Pure Grade): The content of most impurity elements is less than 10 ppb, which is suitable for the daily analysis work of atomic absorption spectrometer (AA).
