
Novices to UV/EB polymerization will quickly discover that most oligomers used in energy-curable formulations have very high viscosities, typically in excess of 1×10 centipoise (cP). A typical example of such a high viscosity material is an acrylate functional epoxy oligomer based on bisphenol A diglycidyl ether (DGEBA).
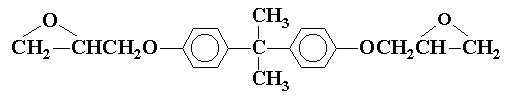
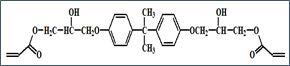
Figures 1 and 2 depict the molecular structures of DGEBA and its diacrylate, respectively. This simple process of reacting DGEBA with acrylic acid increases the viscosity of the material by a factor of almost a thousand!
What is the main reason for the large increase in viscosity after acrylate functionalization of DGEBA? The answer involves the dramatic increase in intermolecular attractive forces after the acrylate step.
DGEBA is a relatively low polarity compound, mainly composed of a non-polar aromatic core structure, two weakly polar ether bonds, and two ethylene oxide (epoxy) structures. The difference in electronegativity between an oxygen atom and the carbon atom to which it is bonded creates a relatively weak dipole-dipole attraction between molecules.
And, like all atomic, ionic, and molecular species, the compound also exhibits instantaneous dipole-induced dipole attraction, or “London dispersion forces” (LDFs). These attractive forces result in DGEBA having a relatively high viscosity for liquid substances of about 12,000 cP, which is about 12,000 times that of water!
DGEBA’s diacrylate contains the same non-polar hydrocarbon core as its epoxide-functional starting material, but it also contains two rather polar acrylate end groups.
In addition, more importantly, the compound contains two highly polar secondary hydroxyl groups (-OH). These -OH groups create a very strong attraction between molecules – a force known as hydrogen bonding. These hydrogen bonds are the main reason for the significant increase in viscosity observed.
Oligomers are typically the main or base component of any UV/EB polymerization formulation and are selected by the formulator to match the key properties required by the end application.
For example, if an aliphatic urethane oligomer is required for a particular general end use, UV/EB formulators will choose an aliphatic urethane acrylate oligomer as the starting point for formulating the product.
Ideally, oligomers may be used neat without dilution. However, its high viscosity is a problem for both manufacturers and formulators. This requires the addition of other components to reduce its viscosity.
In traditional coatings, inks, and adhesives, the viscosity of oligomers or resins tends to decrease with the addition of various organic solvents. However, this is not desirable for UV/EB polymerization due to the negative environmental impact and inherent flammability of volatile solvents.
Therefore, it is known that non-volatile, non-flammable functional monomers that can be copolymerized with oligomers during energy curing are used to reduce their viscosity to acceptable levels. These monomeric diluents remain in the final product and do not evaporate.
But this creates a situation where the choice of monomer can be complicated by the fact that the monomer has a significant effect on the performance of the end-use product. If a simple, inexpensive method could be developed to reduce the effect of hydrogen bonding on the viscosity of oligomers, then a truly usable product could be obtained with significantly lower monomer concentrations.
What is the approach to this problem?
Student researchers in the laboratory of the Center for Applied Polymer Science Research (CASPR) at the University of Houston conducted experiments to find a relatively simple and inexpensive way to reduce the viscosity of this oligomer without the use of monomers.
This work began with a research investigation into the claims of U.S. Patent 4,687,8061. The patent, granted to Interez Corporation (now a business unit of allnex) in 1987, claims that very small amounts of aqueous lithium bromide [LiBr(aq)] can significantly reduce the viscosity of bisphenol A epoxy diacrylate.
The premise of this work is the assumption that lithium ions (Li+) should have suitable size and charge density to interrupt hydrogen bonding. The main formulations tested contained various mixtures of 98% oligomer by mass and 2% by mass deionized water and lithium bromide, and the optimal ratio was found to be 1.85/0.15 deionized water/lithium bromide.
While the researchers found that the lithium bromide aqueous solution did significantly reduce the viscosity of the oligomers, they also found that pure deionized water was more effective at reducing viscosity than any lithium bromide aqueous solution.
Their results were reported at the Photopolymerization Fundamentals Conference in Breckenridge, Colorado in 2011 and the RadTech UV+EB Expo and Conference in Chicago in 2012.
Figure 3 shows the effect of increasing lithium bromide concentration on the viscosity of oligomer/lithium bromide (aqueous) mixtures. It can be seen that the viscosity of the solution containing 0.15 mass % lithium bromide is nearly 90% higher than that of the formulation containing only deionized water. Furthermore, the viscosity continued to increase in a substantially linear fashion with increasing lithium bromide concentration. The hypothesis that Li+ breaks hydrogen bonds seems to have been rejected.
In fact, in separate experiments, adding 2% by mass of lithium bromide to pure oligomers without any deionized water produced a highly viscous elastic liquid that could stretch somewhat like a rubber band. This suggests that Li+ acts as an ionic crosslinker rather than disrupting hydrogen bonds!
To test this, when deionized water was added back to the elastomer mixture, the viscosity was reduced accordingly. The hydration of Li+ ions clearly leads to the breakdown of the ionic crosslinks.
For the pure oligomer sample used in the experiment shown in Figure 3, simply mixing 2% deionized water reduced its viscosity by about 89%.
The researchers then tried to determine the compatibility limit of deionized water with the oligomer. They found that deionized water and oligomers were completely miscible, up to 4% by mass. When the ratio reaches 5%, a cloudy heterogeneous mixture is observed.
Interestingly, if it is assumed that two water molecules are required to break the hydrogen bond between two oligomer molecules, the water/oligomer mass ratio is about 3.7% – close to the 4% observed in experiments .
How does deionized water dilute compared to acrylate functional monomers? Octyl/decyl acrylate (ODA) and 1,6-hexanediol diacrylate (HDODA) are lower DGEBA-based diacrylate oligomers, according to work presented at the Radcure ’86 conference in Baltimore, MD The two best monomers for viscosity.
With this in mind, the viscosity-reducing ability of deionized water was compared with that of ODA and HDODA. Figure 4 shows that the viscosity of deionized water at a concentration of only 2% (mass) is significantly lower than that of 4% ODA or HDODA!
Obviously, deionized water is a very effective and inexpensive oligomeric viscosity reducer.
